Http Phet Colorado Edu en Simulation Molecules and Light
Collect your molecules and view them in 3D. Recognize that the coefficient indicates the total number of molecules.
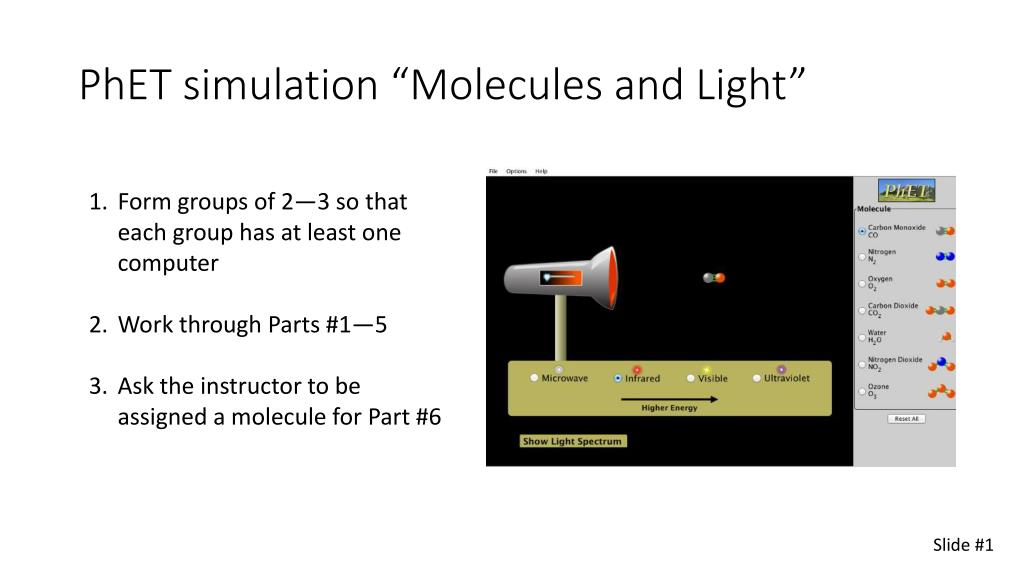
Ppt Phet Simulation Molecules And Light Powerpoint Presentation Id 5618735
This task encouraged students to reflect on the purpose of the simulation before moving on to answer questions regarding its content.

. Microwave Infrared Visible Light Ultraviolet CO N 2 O 2 CO 2 H 2 O NO 2 O 3 Effect 7. Explore bending of light between two media with different indices of refraction. The simulation demonstrates how light interacts with matter on a molecular level.
Collect your molecules and see them in 3D. View the microwave field as a wave a single line of vectors or the entire field. Imagine a molecule with 7 electron domains.
See how changing from air to water to glass changes the bending angle. I 3-2 I atoms and 3 lone pairs on central I 15. How does speed and size of molecules compare to speed and size of familiar objects.
Relate the energy of the light to the resulting motion. A First use the Visible Photon light source eg. The last molecule CCl3F is not in the Molecule Polarity simulation but was included because it is reactive with UV light and is a notorious CFC.
Construct simple molecules from atoms. What units are used to measure temperature and pressure. This prompt helps students to connect light used in the simulation to real light which follows Ehcλ.
Predict the motion of a molecule based on the type of light it absorbs. 2 Importance of Spectroscopy and Photochemistry II Absorption of solar and earth radiation by atmospheric molecules directly influences the energy balance of the planet Greenhouse effect CO2 H2O N2O CFCs Stratospheric temperature inversion O 3 photochemistry Spectroscopy of atmospheric molecules is used to detect them in situ OH is detected via its. Sample Learning Goals Describe matter in terms of molecular motion.
EXPLORING INTERACTIONS OF MATTER WITH LIGHT 3 PART 4. This prompt helps students to connect light used in the simulation to real light which follows Ehcλ. Predict the motion of a molecule based on the type of light it absorbs.
Molecules and Light-inquiry for high school. Light Atoms - Light and Atoms PhET Go to httpphetcoloradoeduensimulationbeers-law-lab Open up the Beers Law Lab simulation. OF 2 2 F atoms 2 lone pairs on O d.
PhET Interactive Simulations University of Colorado Boulder httpsphetcoloradoedu If your use includes redistribution of the simulations please let us know with this form. Identify that absorption of light depends on the molecule and the type of light. Relate the energy of the light to the resulting motion.
Sample Learning Goals Describe the differences between an atom and a molecule. Steps to perform with simulation are in BOLD questions to answer are in ITALICS. View the light as a.
Make a whole rainbow by mixing red green and blue light. Do you ever wonder how a greenhouse gas affects the climate or why the ozone layer is important. Molecules and Light - Guided Inquiry Activity.
The last molecule CCl3F is not in the Molecule Polarity simulation but was included because it is reactive with UV light and is a notorious CFC. Use any resources required to add names to all the geometries in the table on page 3. Then summarize the effects of each type of electromagnetic radiation on the molecules.
Description How do microwaves heat up your coffee. MOLECULES IN THE SIMULATION The interaction of light with a molecule depends on characteristics of the molecule. Permission is granted to freely use share or redistribute PhET sims under the CC-BY license.
Open the Molecules and Light simulation from PhET. Adjust the frequency and amplitude of microwaves. Molecules and Light - PhET Interactive Simulations.
Add 100 N2 molecules to the box. Certain molecules react with each kind of light. Adjust light source slider and begin your observations of how different molecules react to different light sources.
Change the wavelength of a monochromatic beam or filter white light. Note that the interactive elements in this sim have simple description that can be accessed using a screen reader. Identify that absorption of light depends on the molecule and the type of light.
Move the slider in the middle of the flashlight until you start seeing photons coming out of the flashlight. This resource is optional and provides nice visuals of the molecules listed above but some students may not need this tool. Starting from atoms see how many molecules you can build.
Watch water molecules rotating and bouncing around. Answer the questions in your Student Activity Sheet. Use the sim to explore how light interacts with molecules in our atmosphere.
Using PhET in High School Chemistry- all my activities in pdf. Right hand side and add the molecules as shown in the image below. This resource is optional and provides nice visuals of the molecules listed above but some students may not need this tool.
The following attribution is required. Explore how light interacts with molecules in our atmosphere. Certain molecules react with each kind of light.
PF 3 3 F atoms 1 lone pair on P c. Identify that energy increases from microwave to ultraviolet. The geometry is called pentagonal bipyramidal.
Identify that energy increases from microwave to ultraviolet. To do this go to the window next to Heavy Species. Explore how light interacts with molecules in our atmosphere.
Recognize that the subscript in the molecular formula indicates the number of that atom in the molecule. Demo CQs Lab HW. This task encouraged students to reflect on the purpose of the simulation before moving on to answer questions regarding its content.
Molecules And Light Molecules Light Photons Phet

No comments for "Http Phet Colorado Edu en Simulation Molecules and Light"
Post a Comment